In early summer, emergency departments in several regions report a noticeable increase in patients presenting with fever, headaches, and neurological symptoms. Weeks earlier, veterinary professionals had observed a rise in tick-borne infections in wildlife. Separately, environmental analyses an unusually mild winter followed by a warm, humid spring.
Each of these signals was captured, analyzed, and documented — but in different systems, by different institutions, and largely isolated from one another. What could have been recognised as an emerging pattern weeks earlier only became visible once it translated into increased clinical workload.
This example makes the One Health concept tangible: health does not begin in the clinic. Growing evidence of interactions between environmental stressors, animal health events, and human disease has firmly anchored the One Health approach in science and regulation. What has long found its way into guidelines, strategy papers, and funding programmes often still fails in practice — because of infrastructure. The underlying data streams remain fragmented to this day: technically, organisationally, and diagnostically.
And yet, this is exactly where a major opportunity lies. If data from different domains are not only collected, but meaningfully connected, risks can be identified earlier, diagnoses interpreted in context, and prevention made more effective.
Fragmented diagnostics in a connected world: where classic diagnostics reaches its limits
Environmental monitoring, veterinary surveillance, and public health analytics often run in parallel to (individual) clinical diagnostics — and typically follow their own internal logics:
Environmental analytics, veterinary diagnostics, and human medicine operate in separate systems
Classic LIS/LIMS solutions are primarily designed for human clinical workflows
Contextual information on environmental exposures or animal health rarely informs clinical decisions
The result: relevant early indicators remain unused, links between exposure and disease stay invisible, and preventive measures often begin only once clinical impact is already evident. In the context of climate change, urbanisation, and global mobility, this fragmentation becomes increasingly problematic.
What is missing is an integrated diagnostic infrastructure that systematically connects non-human samples with clinical diagnostics — and derives explainable, action-relevant insights from this combined view.
The vision: a One Health Intelligence Platform
A One Health Intelligence Platform rethinks diagnostics: as a shared system for environmental, animal, and human samples. It acts as an LIS for non-human sample types while simultaneously bridging into clinical care and public health.
The goal is not another monitoring tool, but a diagnostic reference system that:
represents and cross-contextualises environmental, veterinary, and human medical data within a shared data model
enables early-warning and risk models aligned with One Health — instead of purely reactive evaluation
provides stronger decision foundations for laboratories, hospitals, and public health authorities
One Health turns isolated measurements into a coherent health picture. Health is no longer assessed in isolation, but systemically — evidence-based, interoperable, and scalable.
Practical examples: why diagnostics must become cross-sectional
One Health is not a theoretical construct. Many current health challenges already sit at the intersection of environment, animals, and humans. Examples include:
Vector-borne diseases and shifting ecosystems
The geographic spread of tick-borne diseases such as Lyme borreliosis or TBE (tick-borne encephalitis) in Europe is closely linked to climate change, land use, and changes in wildlife populations. Rising temperatures and shifting habitats allow vectors to establish themselves in new regions. Veterinary findings in wildlife and livestock often precede increases in human cases — yet they are rarely integrated systematically into clinical diagnostics.
Wastewater signals before clinical outbreaks
Wastewater monitoring has shown that viral circulation can often be detected days or weeks before case numbers rise in clinics. Similar approaches are becoming increasingly relevant for influenza, noroviruses, or resistance genes, providing early situational awareness that individual testing alone cannot deliver.
Antimicrobial resistance beyond the hospital
AMR does not emerge exclusively in clinical settings. Antibiotic residues, resistant bacteria, and resistance genes can be detected in wastewater, surface waters, animal farming, and wildlife. Environmental and veterinary data often reveal new resistance patterns earlier than clinical antibiograms.
From classic LIS to a One Health platform
At its core, the platform extends familiar LIS functionalities — order entry, reporting logic, reference ranges, and quality control — to new sample types and data sources. These are combined with AI-based analysis and forecasting models.
Data points: what flows into the platform
-
Environmental samples: water, soil, air, wastewater, bioaerosols
-
Veterinary samples: wildlife and livestock
-
Human medical data: laboratory and routine clinical data
-
Context data: climate, geography, emissions, demographic parameters
All information is transformed into a shared, interoperable data model and evaluated using explainable AI.
Modular building blocks: from separated data to shared decisions
On this common data foundation, the platform provides modular analytical and diagnostic capabilities. These modules define how data from one or multiple sources are combined, interpreted, and translated into actionable insight.
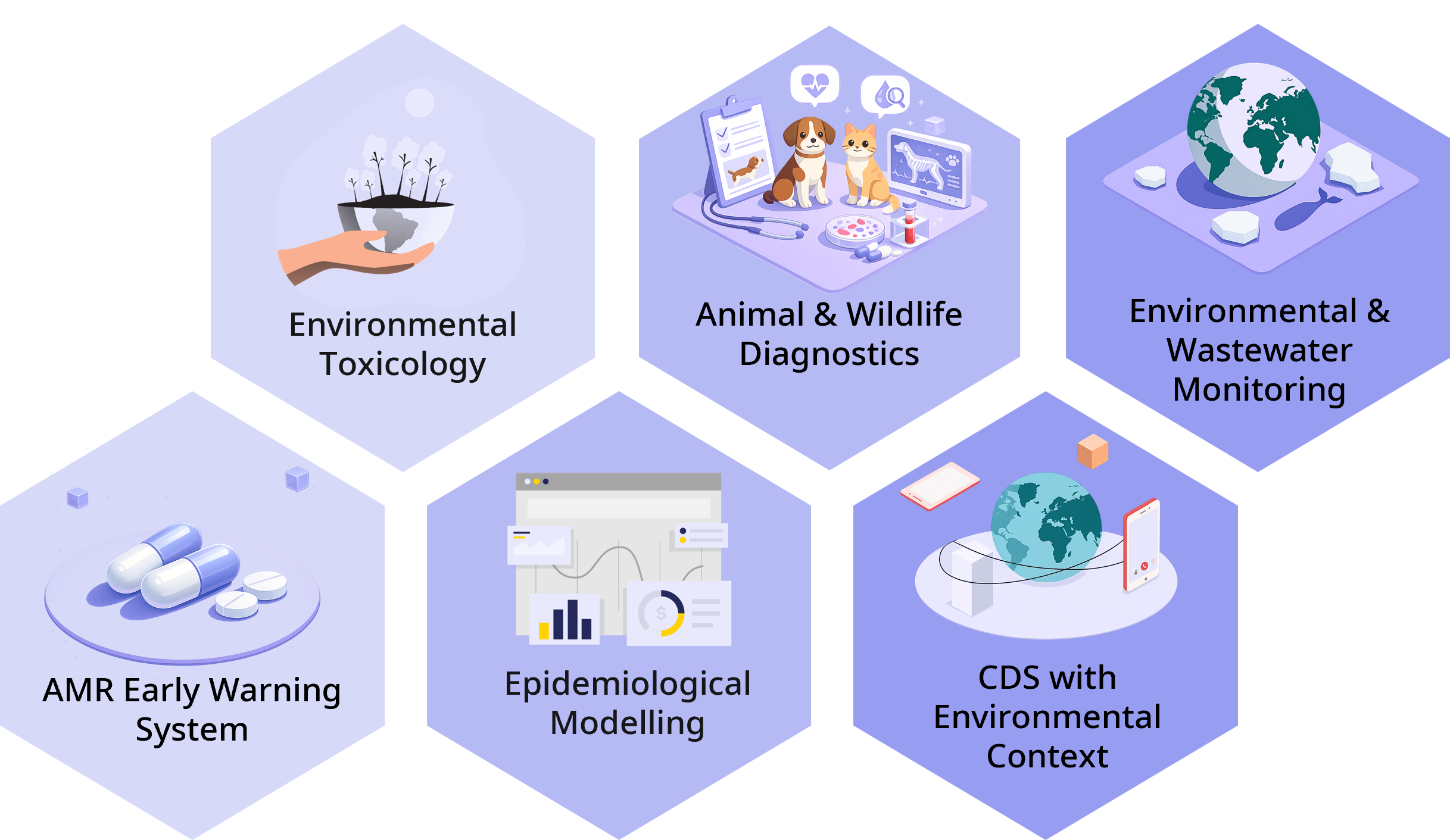
To remain flexible and scalable, the platform is modular by design. Modules can be deployed independently or combined step-by-step. Key modules include:
-
Environmental toxicology with a human clinical focus
Classic lab diagnostics is expanded with an environmental medicine perspective. Pollutants such as PFAS, heavy metals, or pesticides are analyzed in blood and urine samples and contextualized by region, occupation, environmental factors, and comorbidities. The result: structured, AI-supported risk profiles for hospitals, occupational medicine, and public health authorities. -
Animal and wildlife diagnostics as an early-warning system
Zoonotic pathogens do not respect sector boundaries. Integrating veterinary laboratories and wildlife monitoring enables spatiotemporal analyses of pathogens such as TBE viruses, Borrelia, or Leptospira — including structured interfaces to public health systems. -
Environmental and wastewater monitoring
Environmental and wastewater analytics becomes a continuous data source for population-level monitoring. Pathogens, resistance genes, and pharmaceutical residues are aggregated, trends identified, and anomalies detected early — often long before clinical care systems see the impact. -
AMR early-warning system
AMR does not arise only in hospitals. Comparing resistance genes across environmental, animal, and clinical samples enables the early detection of emerging patterns and supports regional antibiotic stewardship programmes more precisely. -
Epidemiological modelling
AI-based models analyze relationships between environmental conditions and disease burden. Forecasts of infection waves, heat or particulate matter effects, and scenario simulations provide a robust decision basis — including transparent uncertainty quantification. -
Clinical decision support with environmental context
Insights are fed back into existing clinical workflows. Examples include pneumonia clusters linked to Legionella burden, respiratory disease interpreted in light of pollen and particulate matter data, or unclear symptom patterns supported by environmental toxicology signals.
What this means for everyday diagnostics
An integrated One Health approach directly impacts routine diagnostic work. Environment-related exposures detected in human samples can be contextualised by region and occupation. Veterinary findings become structured early-warning indicators, while wastewater and environmental monitoring adds a population-level lens to individual diagnostics.
Resistance patterns can be tracked across domains, and clinical decision support is enriched with relevant external evidence — without adding complexity for end users.
Detect earlier, respond sooner: how different stakeholders benefit
The concrete value differs by stakeholder group:
For hospitals
additional context for diagnostics and differential diagnoses
earlier signals of regional exposure events or outbreak dynamics
improved preparedness for care and capacity peaks
For public health authorities
earlier, data-driven situational awareness across environmental, animal, and human indicators
stronger foundations for prevention and intervention measures
support for surveillance, risk assessment, and communication
For environmental and veterinary laboratories
expanded diagnostic relevance beyond a single sector
new application fields for existing analytics
integration into broader health and early-warning systems
For research, municipalities, and companies
access to structured, cross-domain real-world data
evidence-based foundations for environmental, health, and preparedness decisions
support for planning and impact evaluation
Conclusion
One Health does not fail because of missing knowledge — it fails because of missing connections. The practical examples are already reality. What is still lacking is an infrastructure that brings environment, animal, and human health together in a systematic, action-oriented way. A One Health Shared Intelligence Platform does exactly that — not as a theoretical concept, but as a modular, extensible diagnostic operating system.
This is not about reinventing diagnostics. It is about connecting what is already measured — and making it usable for everyday diagnostic decision-making.
The next step is therefore not another study, but the focused implementation of shared diagnostic infrastructures that enable laboratories, hospitals, and public health authorities to translate One Health insights into real-time action.