Most Laboratory Information Systems (LIS) have been built over the years resulting in an ever-increasing complexity with partly overlapping services, often customized processes, and multiple point-2-point connections. Thus, there remains significant room for improvement regarding the IT infrastructure and architecture to ensure stable processes while, at the same time, enabling the greatest degree of flexibility for innovations and the increasing demands on modern IT systems.
System complexity hinders efficiency and innovation in healthcare
The complexity of the systems limits the efficient use of its functionalities and data: Information often needs to be maintained redundantly, synchronization of analysis data and patient data is only possible to a limited extent due to the lack of interfaces, and although most processes are already digitally mapped, a lot of manual work is still required at the workflow level. Decisions (both at the corporate and patient level) cannot be made and executed in real-time. Even routine tasks (e.g., onboarding new senders or obtaining customer feedback) are often opaque and not automated. If there is a desire to introduce new functionalities, these are often complex to implement, dependent on the manufacturer (vendor lock-in), and expensive (e.g., due to missing interfaces). Due to the inertia, it is difficult to respond quickly to changing external and internal influencing factors.
Current approaches and their barriers
Given these challenges, there is an increasing demand for new standards for keeping LIS up-to-date and adapting to internal and external challenges.
We often see two options that IT vendors are promising:
Buy another point solution (e.g., a new order entry)
Move the existing system into the cloud
While generally, both solutions might help for specific aspects, more is needed to address core IT-architectural challenges. Adding a new “Front-End” to your LIS (new Order Entry) still does not address the challenge of multiple point-to-point integrations, whereas moving a “monolithic” and highly individualized LIS to the cloud does not give you monthly updates or the agility the cloud typically promises.
How efficient LIS optimization can look Like
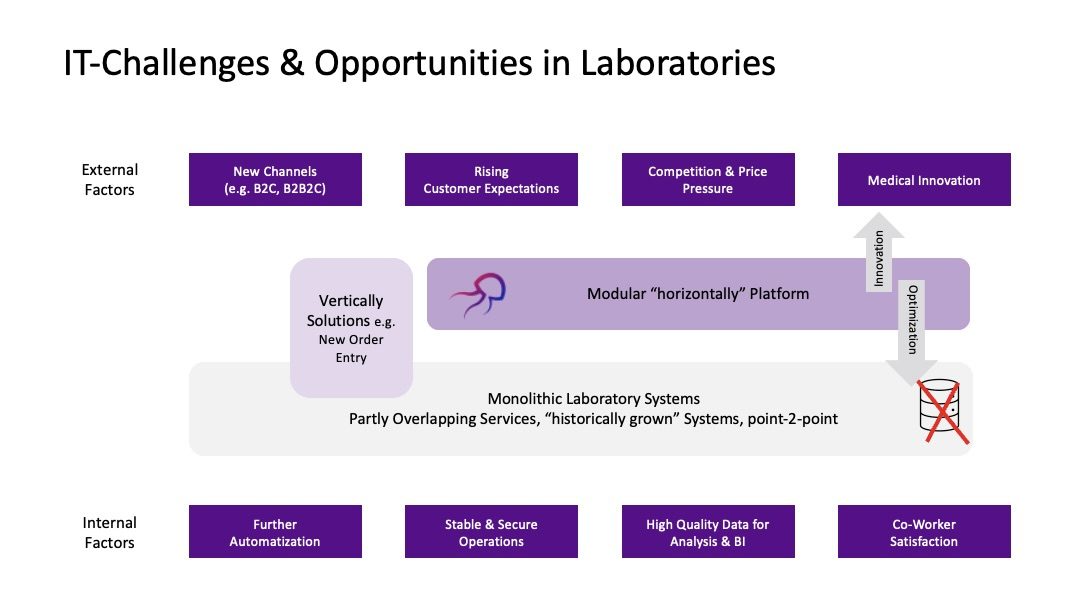
We recommend taking a step back and evaluating the IT architecture and process needs. Often, there is an excellent opportunity to move towards a more modular architecture. Instead of adding more functionality to the existing LIS, one should define the core LIS processes and integrate other parts in other modules.
Firstly, it is necessary to consider which data actually need to be maintained and managed within the LIS. While the LIS will continue to be the leading system for lab tests, for instance, there is no necessity to incorporate all pre-analytical information into the LIS data. Here, there’s also the possibility to look at solutions from other industries (e.g., ERP and CRM functions). In both systems, customer information is required, where ERP manages certain information such as billing, while CRM contains marketing-relevant information.
Another aspect to consider is the system and data interoperability. Another aspect to consider is the system and data interoperability. By using open interface standards (such as FHIR) and introducing internationally recognized standards for medical data (e.g., LOINC, SNOMED CT), effective communication between systems, without having to invest in often overpriced interfaces, can be enabled. Additional information on this topic can also be found in our blog article: “The Use of LOINC Standards in the DEMIS Electronic Reporting System“.
However, we see the primary advantage of the modular approach in the potential for more innovation and the avoidance of a vendor lock-in. While the LIS remains lean and focused on core processes, other functionalities can be outsourced to complementary modules. For instance, intelligent workflow and data management can be incorporated, which automates and controls process steps (e.g., the digital implementation of SOPs) and facilitates routine tasks like onboarding new customers. In addition, there is a central order management that combines integrated laboratory requisition and reporting in one tool, potentially ensuring consistent and secure order processing of intelligent and aggregated orders and results (POCT, in-house lab, external referral…).
We enable agility through modularization
At medicalvalues, we provide a horizontal platform which is built on top of the preexisting LIS and adjusted based on individual demands. This means:
- Individual solutions instead of Vendor-LockIn
- Platform independence instead of isolated systems
- Integrate internationally recognized standards (e.g., LOINC) and FHIR interfaces
- Intelligent Workflow & Data Management
- Central Order Management
Implementing modular systems can help laboratories overcome the limitations of LIS without having to rebuild it. At medicalvalues, we can to review the architecture and processes of your platform and offer a simplification and modularisation to be more agile in the long term.