The Value and Limitations of Laboratory Information Systems
Data plays an integral role in all aspects of a modern laboratory, from order management, test tracking, and reporting to quality control, cataloging, and communication with partners and clients. Medical data is expected to become even more complex and abundant in the future, as more sophisticated testing and diagnostic methods are developed. With the increasing importance of incorporating data into the diagnostic process and laboratory workflow, Laboratory Information Systems (LIS) have become essential infrastructure in all medical institutions.
LISs are implemented to track, organize, and communicate relevant information, making them crucial to the efficiency and effectiveness of any laboratory, regarding both the internal workflow and the partner and user experience. However, the configuration and use of modern LISs remain complex, error-prone, and time-consuming, while the full potential of laboratory data often goes unrealized. To address this issue, medicalvalues offers an extension of classical LISs that aims to improve laboratory processes and enable better utilization of available resources.
The medicalvalues Diagnostic Data Management (DDM)
The medicalvalues Diagnostic Data Management System is built around a centralized database, also known as a “Single Source of Truth”, which contains all the relevant information on the laboratory’s available analyses. To ensure that the stored and shared data is consistent, accurate, and up to date, the medicalvalues operating principle follows a scheme of extracting or updating information, enhancing it, and sharing it with the relevant parties. At the same time, this approach reduces the manual effort and time required to synchronize information between different applications and safeguards the system against human error during this process. In the following sections, we will explore these steps, as well as their integration into the existing process chain of laboratory datasets.
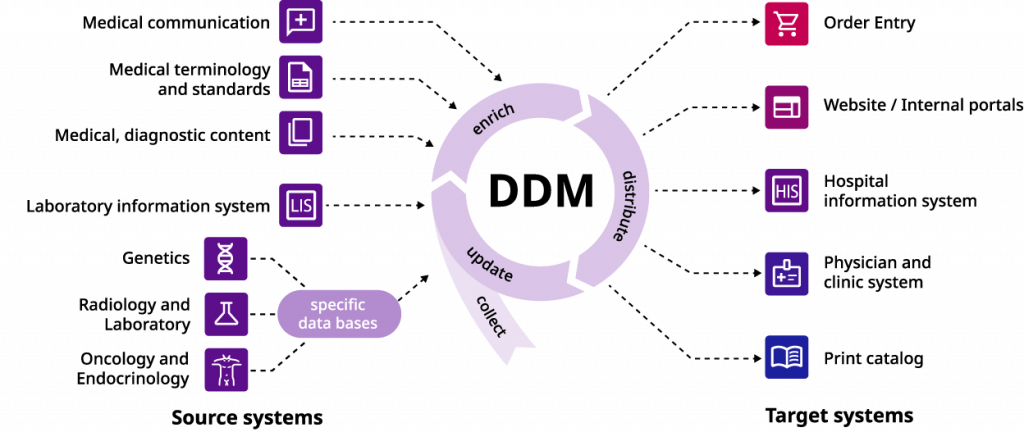
Extraction of data from different source systems
To build and operate a centralized database, the system must be capable of importing data from different sources. This involves not only the initial integration of the laboratory’s existing datasets but also additional sources that become relevant during operation to keep the system up to date. These sources include the existing Laboratory Information System (LIS) of the laboratory, as well as specialized external databases in fields such as radiology, oncology, or genetics.
Another vital aspect of a centralized database is the ability to edit and revise stored data. In order to ensure the laboratory information remains up-to-date, the system allows for the addition of new analytes or devices, changes to the workflow (e.g., the possibility for parameters to be measured in-house), and automatic adjustments to individual channels. The optional integration of the (semi-)automated medicalvalues Diagnostic Data Mapper in the system also enables intelligent LOINC coding of the datasets, thus facilitating interoperability between different systems.
Processing/enrichment of the data
Rather than simply storing medical information in a centralized database, the medicalvalues DDM allows users to enhance imported data with various medical and marketing-relevant information. The documented analyses can be enriched with details such as translations, descriptions, or billing information to make it more understandable to the respective target group (e.g., third parties, employees, and partner laboratories or customers). Lastly, documented medical insights relevant to the stored data can also be enclosed, ensuring that valuable information is available to users at the appropriate place and time. These enhancements to the original dataset can have a tangible impact, increasing both the usability and the value of the laboratory’s datasets.
Distribution & visualization of target group-relevant information
One of the principal functions of a centralized database is the ability to share information with partners and third parties. The DDM system allows for the linking of stored information through the creation of portfolios or collections, enabling relevant datasets to be allocated to specific target channels. Examples of external channels for which sharing specific sets of analyses would be relevant include order entry, the institution’s website or internal portal, hospital information systems, medical and clinical systems, or a print catalog of the laboratory’s competencies. In all these scenarios, the added structure and categorization of the central database can make accessing the required information safer, easier, and more practical. At the same time, this approach saves valuable time and effort that would otherwise go into separately updating the relevant communication channels.
Direct benefits of Diagnostic Data Management with medicalvalues
Enhancing existing LISs with the capabilities of the medicalvalues DDM can yield significant benefits for the internal workflow of the laboratory, as well as for its capacity to offer a practical, clear, and efficient experience for its clients.
The DDM incorporates features that improve and simplify the workflow, automate standardized tasks, and simplify complex tracking processes. This frees up resources by unburdening personnel from executing time-intensive tasks, while safeguarding the system against human errors that could occur during such processes.
The DDM serves as a Single Source of Truth for all relevant diagnostic data. As all further applications and services receive information from one centralized database, data consistency across all channels can be ensured without the need to manually update the information in each channel. This means that the data shared with coworkers, partners, or clients will be accurate and relevant, whether it is in a catalog, order entry system, service specification webpage, or other related channels.
Lastly, the possibility to customize portfolios or collections of analyses enables users to personalize and adapt the displayed data according to the needs of their customers. This can also extend to include customer-specific price information and additional marketing details. By using the medicalvalues DDM, you can provide a personalized experience for your clients and create an important competitive advantage.
Key system features
To achieve these benefits, medicalvalues incorporates a range of software features that can improve the experience of both workers and customers. Below are some of the key features:
The DDM system also includes collaboration and approval functions, enabling medical, product management, and marketing teams to work together seamlessly. The integrated user management ensures that only authorized personnel can access and edit the information.
medicalvalues provides pre-built integrations and advanced API functionality, including the ability to connect different laboratory (sub-)systems, to enable the seamless implementation and scaling of the DDM system. This feature streamlines the process of data sharing and ensures that information is accessible to all relevant parties.
The integrated comprehensive information enrichment function allows users to add and update textual information, images, videos, translations, and commercial information. This feature provides a more detailed and comprehensive overview of medical information, making it easier for users to understand.
The platform’s flexible customization options enable laboratories to configure custom data types and relationships without the need for programming knowledge. This feature ensures that the platform can adapt to the specific needs of each user.
The DDM provides diagnostic content templates that allow users to customize their diagnostic content for publication on their own or a medicalvalues-based website. This feature provides a simple and efficient way to share medical information with patients, partners, or clients.
The integrated (semi-)automated data mapping allows for the conversion and mapping of data according to international standards such as SNOMED CT and LOINC. This is an essential step towards interoperability, as it ensures that the information provided is consistent and easily understandable across different systems and countries.
The big picture
In addition to the benefits of a Single-Source-of-Truth-approach discussed above, it is important to consider the role of a centralized database in the broader context of modern laboratory settings. This raises further requirements for the proper integration of the DDM but also unlocks new opportunities for users to fully leverage the potential of their clinical database. For instance, the (semi-)automated medicalvalues Diagnostic Data Mapper can efficiently cover the need for dataset standardization before importing them into the database. Furthermore, connecting the diagnostic data management to the medicalvalues Diagnostic Intelligence can provide valuable diagnostic insights into the examined sample, greatly enhancing the diagnostic process. Lastly, the medicalvalues Order Intelligence (order entry system) can significantly improve communication between medical personnel and laboratories, saving valuable time and resources.
If you are interested in learning more about the capabilities of medicalvalues Diagnostic Data Management or other medicalvalues tools and services, please contact us to arrange a live demo.



