Laboratory Reports in the Electronic Health Record
The electronic health record (EHR) enables the secure and cross-sector availability of health data, allowing doctors, hospitals, rehabilitation facilities, and caregivers to integrate important previous findings into their treatment processes and add current information.
As part of this record, the MIO laboratory report ensures that laboratory results are standardized and reliably accessible.
Medical Information Objects (MIOs)
Medical Information Objects (MIOs) can be defined as "small digital building blocks of information" [1] and describe fundamental concepts as well as semantic and syntactic specifications for future digital information exchange in healthcare. After the MIOs for vaccination record, dental bonus booklet, maternity record, and child health record were described in 2020, new MIOs, including the vaccination record, hospital discharge summary, and laboratory report, are now being developed.
The involved institutions agree on the professional content and structures of the respective MIOs, the semantic annotation and coding of the content (terminology systems), and the syntactic implementation in FHIR.
Contents of the MIO Laboratory Report
In the context of defining the MIO laboratory report, a variety of aspects have been discussed. It is decided that internationally recognized standards such as LOINC are used for semantic standardization and identification of laboratory tests, and FHIR for data exchange.
An introduction for clinical chemistry is planned for 2025. Additional modules, such as those for microbiology, are potentially expected from 2026 onwards.
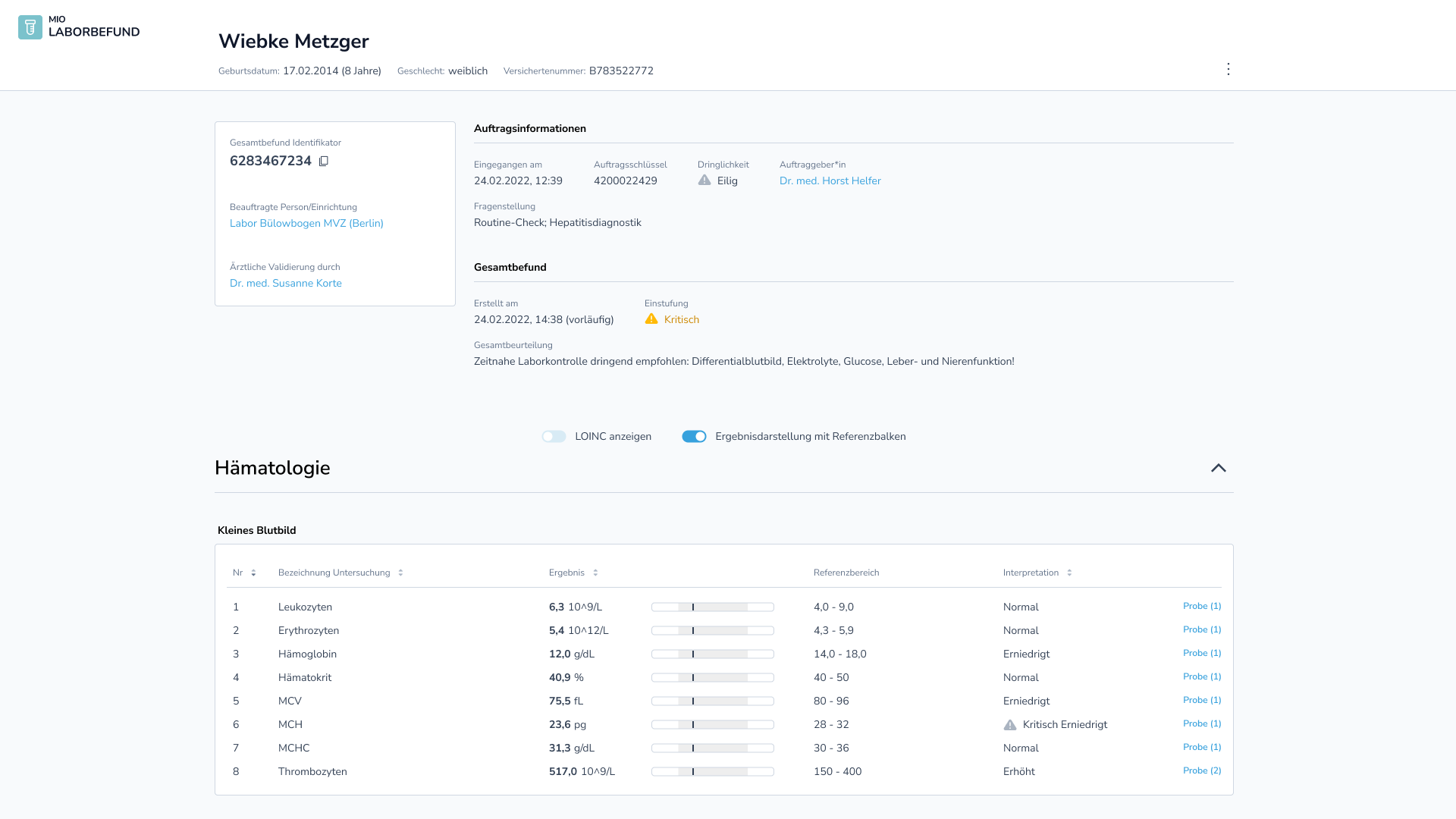
The following figure visualizes an exemplary report [2] according to the MIO standard:
Further details can be found on the official website of the Kassenärztliche Bundesvereinigung (National Association of Statutory Health Insurance Physicians): Projekt-Update – Laborbefund 1.0.0 – MIO
Getting MIO-Ready with medicalvalues
medicalvalues has been actively involved in the MIO working group and has internally made necessary preparations to optimally – and most importantly, pragmatically – prepare you for the upcoming changes.
We offer laboratories consulting and a complete toolkit:
With a connector between LIS (Laboratory Information System) and EHR, we can facilitate laboratory report communication according to the defined MIO standard. Furthermore, we enable secure and bidirectional digital exchange via KIM (communication in medical informatics) messages, serving as a secure and authorized replacement for fax, through connection to the Telematics Infrastructure (TI)
We support with various solutions for the necessary data preparation and harmonization (e.g., simplified LOINC mapping and FHIR)
Let’s work together to identify what is currently necessary for your laboratory to future-proof itself regarding mandatory MIO usage.
Sources:
[1]: https://www.kbv.de/html/mio.php (accessed on 20th June)
[2]: https://mio.kbv.de/display/LAB1X0X0/Visualisierungen (accessed on 20th June)